Incorporating Risk-based Asset Management into your Drug Shortage Prevention Program
By David J. Mierau, PE, CMRP
Risk-based asset management policies and practices for physical assets are valuable additions to operations within the pharmaceutical industry. These practices have strong parallels to commonly used quality risk management (QRM) concepts, and have specific benefits related to preventing drug shortages. Specifically, risk-based asset management programs are intended to control risks associated with product manufacturing, packaging, facilities/utilities infrastructure, and supply chain inventory management within controlled environments.
Quality Risk Management Background
The pharmaceutical industry has been focused on the benefits of risk-based strategies for the past decade, with large-scale guidance on the topic published under ICH Q91. Currently, there are multiple recognized international standards that incorporate these concepts such as ASTM E25002 and the ISPE Baseline Guide for Commissioning and Qualification3. The primary purpose of these standards is to provide requirements and guidance for balancing product quality, patient safety and financial cost. This focus has combined into the overarching concept of quality risk management (QRM) for the pharmaceutical industry.
The driving force for QRM is the need to alleviate the immense burden of validation, qualification and commissioning of systems associated with the development, manufacture and distribution of pharmaceutical products. Traditional methods of paper-based process and equipment validation efforts utilized prior to QRM required pharmaceutical companies to make large investments in time and labor for asset installations and modifications. While these costs are still significant for the industry, they have been reduced. Through QRM, processes that are critical to product quality and patient safety receive the most rigorous validation and care, while other non-critical systems may only require commissioning to ensure basic desired functionality.
While QRM has been a great advancement for maintaining product quality and alleviating some business burdens, it does not expand guidance to other critical aspects of the business such as production reliability and market delivery. To maintain a focus on patient safety but also ensure reliable delivery of products when they are needed, companies need to combine their current QRM policies with risk-based asset management principles. This combination provides the foundation for stabilizing production and supply chain operations, which allows for clear understanding of market delivery capabilities and early warning signs for drug shortages.
Drug Shortages
In October of 2013, FDA published a Strategic Plan for Preventing and Mitigating Drug Shortages in response to a new requirement within the Food and Drug Administration Safety and Innovation Act (FDASIA)4. Due to ongoing drug shortage events reported to FDA and the associated risk to patients, the Agency has been analyzing all facets of reliably delivering pharmaceutical products to the public.
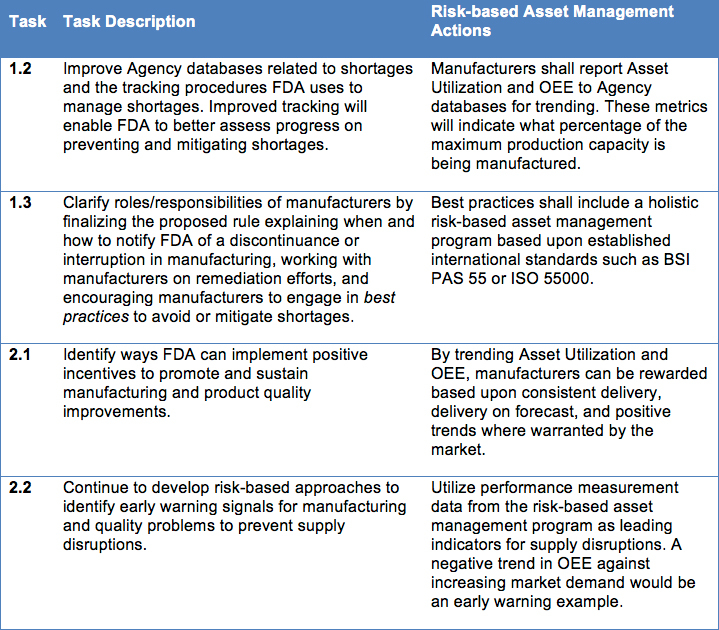
Under the specific goals listed within the Strategic Plan, several tasks were identified to prevent and mitigate drug shortages. The following is an abbreviated list of those tasks that can benefit from risk-based asset management practices:
- Task 1.2: Improve Agency databases related to shortages and the tracking procedures FDA uses to manage shortages. Improved tracking will enable FDA to better assess progress on preventing and mitigating shortages.
- Task 1.3: Clarify roles/responsibilities of manufacturers by finalizing the proposed rule explaining when and how to notify FDA of a discontinuance or interruption in manufacturing, working with manufacturers on remediation efforts, and encouraging manufacturers to engage in best practices to avoid or mitigate shortages.
- Task 2.1: Identify ways FDA can implement positive incentives to promote and sustain manufacturing and product quality improvements.
- Task 2.2: Continue to develop risk-based approaches to identify early warning signals for manufacturing and quality problems to prevent supply disruptions.
The following figure is from the Strategic Plan:
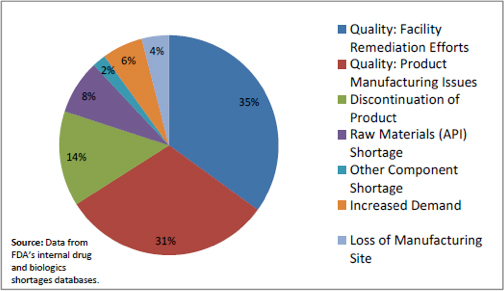
Figure 1: Drug Shortages by Primary Reason for Disruption in Supply in 2012
The data indicates that 66% of disruptions are due to product-specific quality failures (31%) and issues with manufacturing processes or facilities (35%). Risk-based asset management practices can directly address these types of disruptions, and provide a better understanding of production demand concerns.
To further develop information regarding drug shortages, ISPE has developed a Drug Shortage Initiative Task Force to work closely with regulatory agencies and the pharmaceutical business community. The Task Force is comprised of industry representatives and they have been working on ways to better understand the root causes and possible mitigations for drug shortages5. One example of this work is the Drug Shortages Survey issued in February of 2013 to professionals within different sectors of the pharmaceutical industry to collect more specific data regarding contributing factors to drug shortages. The results of this survey have been published by ISPE, and respondents identified that manufacturing quality issues were among the most prevalent causes leading to drug shortages, which is consistent with the information published by FDA6.
Risk-based Asset Management
There are two primary recognized international standards regarding physical asset management: BSI PAS 557 and ISO 550008. The current version of BSI PAS 55 was published in 2008, and ISO 55000 is currently in the final approval stage with expected publication in early 2014. These two standards are providing much-needed structure to the practice of managing physical assets, which is often loosely organized by ad hoc procedures, manufacturer-recommended maintenance practices, and a reactive culture where risks are not proactively analyzed or mitigated.
Both of these standards focus on managing physical assets across their entire life cycle, from concept to decommissioning, with guidance on effective asset management at each stage. The exact same risk assessment and risk management principles utilized by the pharmaceutical industry through QRM are applied within these standards. However, quality is only one component of overall asset criticality in a holistic management approach. Risk management methodologies have been successfully implemented within the pharmaceutical industry that still maintain focus on critical product quality and patient safety requirements, but also incorporate other risk categories such as:
- Safety (internal and external)
- Environmental
- Production Output (quantity)
- Business ($)
- Reliability (Mean time between failure, MTBF)
- Single Point of Failure (lack of continuity or contingency)
- Planned Utilization (%)
- Spare Part Lead Time (days)
- Maintenance Cost
- Decommissioning Cost
- Asset Replacement Cost
The criticality analysis, risk assessment and control strategy development process is just one aspect of risk-based asset management. Figure 2 represents a model for risk-based asset management; note the overall goal of operational stability. Stable operations deliver consistently on schedule with minimal unexpected disruptions. Also, forecasting is much more accurate for a historically stable operation, providing less uncertainty around delivery of products to market.
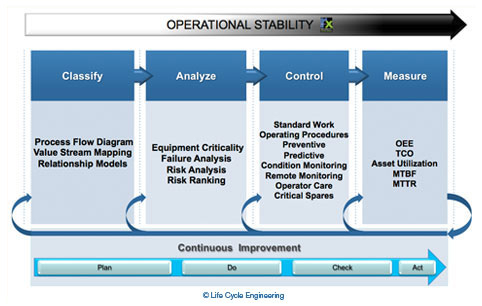
Figure 2: Risk-Based Asset Management Model
The model in Figure 2 also separates activities into four phases: classify, analyze, control, and measure. From an implementation perspective, this progression is ideal to establish the most effective asset management program: one where all risk control strategies are based upon specific asset failure modes. Continuous improvement through monitoring of key performance indicators is also a key feature of this model.
Based upon the structure presented within ISO 55000, other aspects of an asset management program can be organized into the following categories:
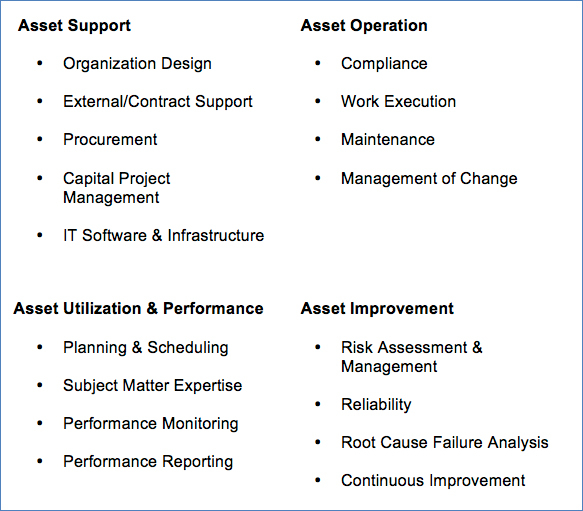
Drug Shortage Prevention Program Structure
While there are many complex aspects of pharmaceutical production and distribution that ultimately must be incorporated into a complete Drug Shortage Prevention Program, Figure 3 represents a high-level structure from the manufacturing organization perspective:
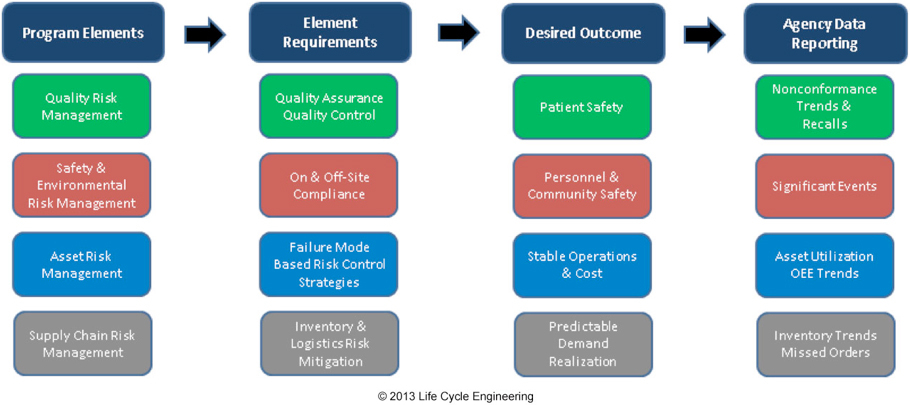
Figure 3: Drug Shortage Prevention Program Structure for Manufacturing
The QRM element is comprised of the traditional Quality Assurance and Quality Control aspects of pharmaceutical manufacturing. Quality Assurance is the standards, policies, procedures, and processes designed to deliver consistent safe, high quality, products. Quality Control is the product sampling and testing division of Quality that exists primarily for intermediate and final product release verifying a product meets safety, identify, strength, purity and quality (SISPQ) requirements.
The Safety & Environmental Element incorporates the aspects of federal and local regulatory compliance requirements. This is an essential component of the Drug Shortage Prevention Program because even though a process may be producing quality product, it could be shut down due to significant safety or environmental concerns that outweigh the impact of stopping production.
Asset risk management, as described under risk-based asset management previously, is a key element to preventing drug shortages because it essentially defines what quantity of product can and will be produced. Holistic asset risk management also incorporates the elements of QRM, Safety & Environmental into implementation as well. For performance monitoring, asset utilization is a fundamental indicator of asset management from a production perspective: this indicates what percentage of assets are functioning over available production time. Overall equipment effectiveness (OEE) is a combination of quality, rate and availability from the operation. While this performance indicator is typically low for the pharmaceutical industry (20-30%), trending this combines multiple aspects of production concurrently and is an excellent high-level monitoring metric.
Supply chain risk management within the manufacturing boundary is a key element of operational reliability as well. Raw products, inventory management and warehousing all have significant potential impact on drug product delivery. The supply chain and asset risk management processes and procedures must be closely aligned to ensure production systems work well together.
Agency Data Reporting as represented in the structure indicates the information that would be included in regulatory agency databases to monitor product delivery. Utilizing a dashboard type of communication, agencies can be notified of negative performance trends in quality, safety, Asset performance or supply chain delivery. Manufacturers would also be monitoring trends prior to data submission, and can provide explanations and action plans where appropriate to address negative trends. Consistent or positive performance that is trending in-line with product demand from the market would not require specific review or explanations.
Conclusion
From the FDA Strategic Plan for Preventing and Mitigating Drug Shortages, the identified tasks can be addressed through risk-based asset management with the following actions:
Preventing drug shortages is about effectively managing risks and utilizing data-based leading indicators to generate warnings early enough to take corrective or mitigating actions. While the overall supply chain will present added complexities from wholesalers, pharmacies, hospitals and healthcare providers, the Drug Shortage Prevention Program structure proposed brings forward key benefits of focusing on risk-based asset management. Improving the reliability of physical assets provides the foundation for operational stability, maintains a focus on product quality and patient safety, while addressing the manufacturing risks associated with drug shortages.
References:
- International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, ICH Harmonised Tripartite Guideline, Quality Risk Management Q9, Current Step 4 version dated 9 November 2005, www.ich.org.
- ASTM E2500-07(2012): Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment, www.astm.org.
- ISPE Baseline Guide Volume 5: Commissioning and Qualification, International Society for Pharmaceutical Engineering (ISPE), First Edition, March 2001, www.ispe.org.
- Public Law 112-144, Section 506C(h)(2) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) 21 USC 356c(h)(2), as amended by Title X of FDASIA, www.gop.gov.
- Information regarding the International Society for Pharmaceutical Engineering (ISPE) Drug Shortage Initiative and Task Force can be found at: http://www.ispe.org/drug-shortages-initiative.
- Berg N, Kos K, et al. Report on the ISPE 2013 Drug Shortages Survey. June 2013. Tampa, FL: International Society for Pharmaceutical Engineering. Available at www.ispe.org/drugshortages/2013JuneReport.
- BSI PAS 55: British Standards Institution Publicly Available Specification 55-1:2008: Specification for the Optimized Management of Physical Assets, www.bsigroup.com.
- ISO/FDIS 55000: Asset Management – Overview, Principals and Terminology, www.iso.org.
David J. Mierau, PE, CMRP is a licensed Professional Engineer and Certified Maintenance and Reliability Professional with a broad range of technical and management experience within the pharmaceutical and biotech industries. He is a member of ISPE and the Society for Maintenance and Reliability Professionals (SMRP).
© Life Cycle Engineering, Inc.

For More Information
843.744.7110 | info@LCE.com
