Line Performance Optimization®: A problem-solving process for pharmaceutical manufacturing
 Line Performance Optimization: A problem-solving process for pharmaceutical manufacturing (PDF)
Line Performance Optimization: A problem-solving process for pharmaceutical manufacturing (PDF)
Line Performance Optimization (LPO) integrates best practices in asset management, lean and change management to solve chronic and persistent reliability issues with a specific manufacturing line. These common, chronic reliability issues include production losses due to unplanned downtime, ineffective maintenance practices, absence of standardized processes, quality-related issues, and loss time due to material flow design.
Organizations using this targeted approach have increased overall equipment effectiveness by an average of five percentage points. The LPO process includes components of Life Cycle Engineering’s proprietary Risk-based Asset Management and Lean Implementation models, along with Prosci’s change management methodology, to address human factors, culture, and process and equipment limitations that adversely impact the value stream.
Here’s a brief description of how LPO can solve issues that are currently plaguing pharmaceutical manufacturing.
Develop a business case
First, you develop a business case to ensure that the optimization effort is warranted from a business perspective. The format for this is an A3 (named for the printer setting for 11” by 17” paper in landscape). The sheet is divided into four quadrants and labeled “Background / Business Case”, “Initial Condition”, “Target Condition”, and “Action Plan and Metrics”. In Figure 1, below, the Background / Business Case quadrant of a completed A3 illustrates the financial benefit of a LPO project reaching the target state. The Implementation and Metrics quadrant shows the activities that are required to move from current state to target state, and the metrics that will be used to monitor the return on investment.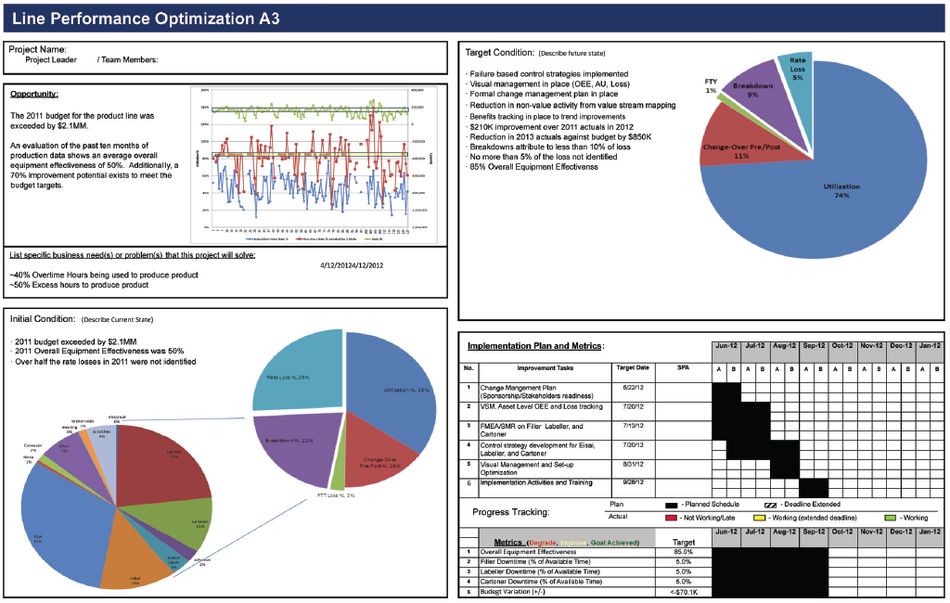
Figure 1: Line Performance Optimization A3
Analyze your process and develop control strategies
Next, you create a value stream map for your manufacturing process. (See Figure 2 as an example of a partial value stream map for a biological drug filling line). Analyze the current-state value stream map and look for ways to improve material flow, eliminate non-value-added activities and optimize manual and automated processes. As part of this analysis, evaluate the mean time between failure and the mean time to repair for your critical equipment to determine if a failure modes and effects analysis is warranted. Then develop your future-state map that reflects the improvements you have identifies.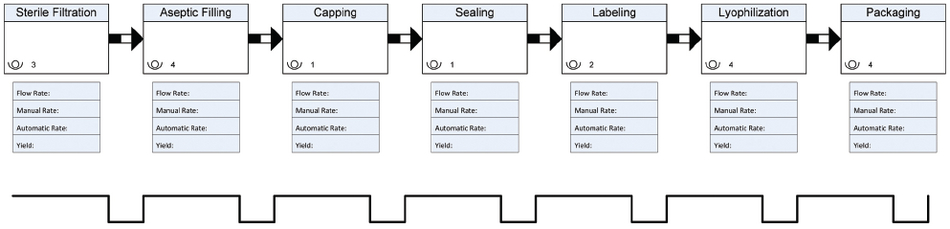
Figure 2: Partial Value Stream Map
Once the equipment has been identified for the failure modes and effect analysis, the key stakeholders perform the analysis by developing a functional block diagram of the equipment, determining the function of each component within the block diagram, and then determining predominant failure modes that have not been addressed by an effective control strategy. The next step is to develop and implement new control strategies such as predictive or preventive tasks, operator care tasks, standard operating procedures, or even stocking a critical spare. Some predominant failure modes may have no control strategy and therefore may become a candidate for a capital project to redesign.
Plan for change
Key stakeholders need to collaborate to develop the formal change management plan for the LPO project. The plan should include a sponsorship roadmap, communication and risk plans, and a readiness for change assessment. This assessment will help identify where communication is needed to help operations and maintenance employees and supervisors understand the need for change. It will also help pinpoint what training is needed to help employees adjust to changing roles and responsibilities.
Once the future-state value stream map has been implemented, you implement loss tracking and a visual management system to ensure that all personnel can see the key performance indicators on line and trending compare actual performance to target performance. Variations below targets can be evaluated and assigned to a loss category, while variations above target can be analyzed to evaluate whether the events that occurred can be duplicated.
Capture meaningful metrics
Overall equipment effectiveness (OEE = uptime x rate x quality) and asset utilization (AU = availability x rate x quality) are two high-level metrics that you can use to measure performance trends. Both calculations for rate and quality are the same. Rate is the average rate over the best demonstrated rate or designed rate. Quality is the first pass yield over the total yield. The difference is availability and uptime.
The loss tracking and metrics you choose to capture provide the transparency to the organization of how you’re performing and where you are experiencing losses. This information makes it possible to follow the continuous improvement process shown in Figure 3.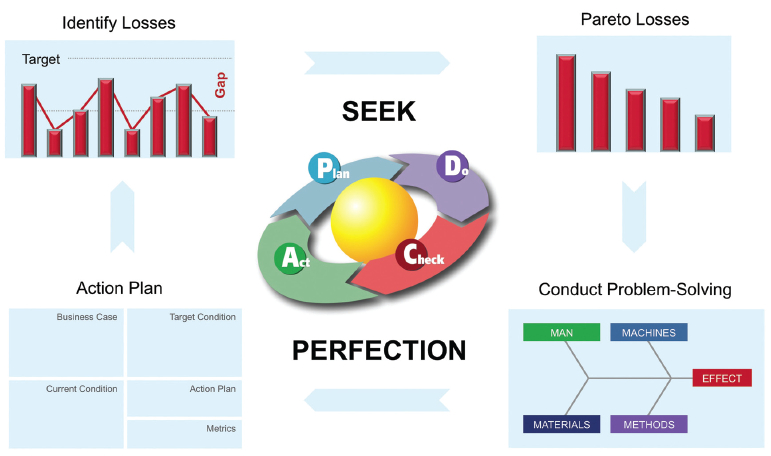
Figure 3: Continuous Improvement Process for Manufacturing Lines
By combining asset management best practices, lean principles and a disciplined change management strategy, Line Performance Optimization creates a unique approach to problem-solving for manufacturers that require meeting strict regulatory requirements as well as adhering to Good Manufacturing Practices. Line Performance Optimization is a time-efficient, targeted solution for chronic reliability issues in pharmaceutical manufacturing that cause quality, downtime, waste and regulatory problems.
© Life Cycle Engineering

For More Information
843.744.7110 | info@LCE.com
